VMS needs a temperature check
More than 70% of
women with
menopausal
symptoms, like
VMS, go
untreated1
Vasomotor Symptoms (VMS), also known as
hot flashes and night sweats, are the
most
bothersome symptoms of menopause. While
the number of women aged
40 to 65 is
projected to grow, many will experience VMS
and remain
untreated.1-3

Research shows that
women want4:
- Credible treatment information
- Facts about diagnosis
- Open and honest conversations
Get the conversation
started
VMS SURVEY
TOOL
Identify frequency and
disruptiveness
NEURONAL
INFLUENCE ON VMS
Watch this expert speaker
session
It’s time to take
VMS off the back
burner
Helping women with VMS has become increasingly difficult and complex over the past 20 years due to patients receiving conflicting information from various sources.4
There has been an 84% decline in hormone therapy (HT) prescriptions for menopause-related symptoms.5
FDA-APPROVED HORMONE
THERAPY Rx VOLUME5*
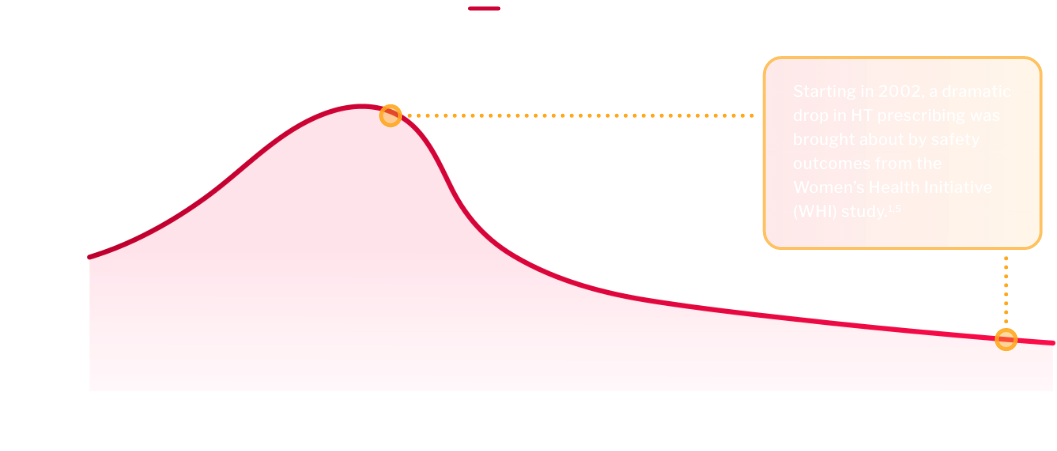
HT remains the standard of care for VMS, but may not be appropriate for every patient6,7
Safety outcomes from the WHI study brought about concerns with HT1,7
Patients may have contraindications for use of HT, and certain considerations like age, onset of menopausal symptoms, and
medical histories need to be taken into account6Patients may have concerns about the risk/benefit profile4
Subsequent analyses of these data have demonstrated that HT is safe and effective when initiated in the appropriate patient at the
right time, formulation, and dose6However, even with these data, prescriptions have not rebounded, leaving some women with minimal options5
Healthcare is trending toward targeted treatment, but VMS are lacking innovation. Other menopausal conditions like vulvovaginal
atrophy (VVA) and osteoporosis have seen targeted innovations; however, VMS have seen very few.4,6,8
FDA-APPROVED HORMONE THERAPY
Rx VOLUME8*

Treatment strategies for VMS
| TREATMENT | SAFETY | EFFICACY |
|---|---|---|
| Estrogen therapy |
| Up to 75% reduction in frequency and 87% reduction in severity |
| Estrogen-progestin therapy |
| |
| Estrogen combined with estrogen agonist/antagonist |
| 74% reduction in frequency and significant reductions in severity |
TREATMENT
Estrogen therapy
SAFETY
- Adverse events include breast tenderness, vaginal bleeding, and bloating
- Includes a boxed warning that refers to stroke, deep vein thrombosis, endometrial cancer,† and dementia‡; estrogen therapy should not be used for the prevention of cardiovascular disease or dementia
EFFICACY
Up to 75% reduction in frequency and 87% reduction in severity
TREATMENT
Estrogen-progestin therapy
SAFETY
- Adverse events include breast tenderness, vaginal bleeding, and bloating
- Includes a boxed warning that refers to stroke, deep vein thrombosis, pulmonary emboli, myocardial infarction, invasive breast cancer, and dementia‡; estrogen therapy should not be used for the prevention of cardiovascular disease or dementia
EFFICACY
Up to 75% reduction in frequency and 87% reduction in severity
TREATMENT
Estrogen combined with estrogen agonist/antagonist
SAFETY
- Adverse events include infection, pain, arthralgia, and headache
- Includes a boxed warning that refers to stroke, deep vein thrombosis, endometrial cancer,† dementia,‡ and use with additional estrogens; estrogen therapy should not be used for the prevention of cardiovascular disease or dementia
EFFICACY
74% reduction in frequency and significant reductions in severity
Other forms of estrogen and progestin may have different risks, such as lower risk of various thromboembolism with transdermal estrogen vs oral estrogen (as indicated by some observational studies). Estrogens
with or without progestins should be
prescribed at the lowest effective doses
and for the shortest duration consistent
with treatment goals and risks for the
individual woman.
† In women with a uterus who use unopposed estrogens.
‡In postmenopausal women 65 years of age and older.
| TREATMENT | SAFETY | EFFICACY |
|---|---|---|
| SSRI§ |
| 33–65% reduction in frequency and significant reductions in severity |
TREATMENT
SSRI§
SAFETY
- Adverse events include nausea, headache, and dizziness
- Includes boxed warning for suicidal thoughts and behaviors in pediatrics and young adults
EFFICACY
33-65% reduction in frequency and significant reductions in severity
§SSRI=selective serotonin reuptake inhibitor.
| TREATMENT | SAFETY | EFFICACY |
|---|---|---|
| Supplements and herbal therapies | Evidence of safety of unregulated products is limited | Inconsistent evidence of clinical benefit more than placebo |
TREATMENT
Supplements and herbal therapies
SAFETY
Evidence of safety of unregulated products is limited
EFFICACY
Inconsistent evidence of clinical benefit more than placebo

VMS IN HER WORDS
“It’s like a snowball [effect]; I don’t
sleep well, then I wake up tired, and
because I am tired, I am irritable.
[Then] I get mad at myself when I am
irritable because I should be patient
with people, especially the ones that I
love.”
VMS IN HER WORDS
“It’s like a snowball [effect]; I don’t
sleep well, then I wake up tired, and
because I am tired, I am irritable.
[Then] I get mad at myself when I am
irritable because I should be patient
with people, especially the ones that I
love.”